Sterility Testing
Advanced microbial contaminant detection and identification via state-of-the-art MALFI-TOF mass spectrometry
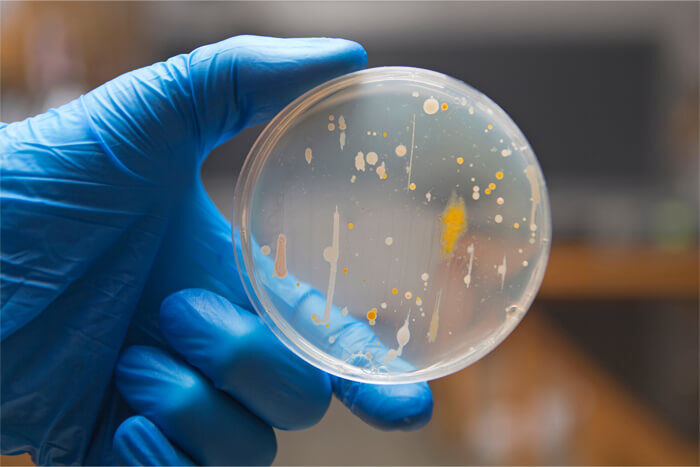
Detect a wide variety of viable contaminants with greater insight than ever
Sterility testing detects and identifies microbial contaminants that can inhibit cell culture performance, contribute to data variability, and threaten animal health. Contamination is not always apparent as visible turbidity or a pH change and can appear in 5-10% of sterility samples.
Using our proprietary blend of eight culture media, we carefully grow nearly any contaminants that affect biological laboratories. Then, MALDI-TOF mass spectrometry delivers accurate species identification from among hundreds of genera of bacteria, giving you the most insight possible as to the source of contamination.
Sterility testing from IDEXX BioAnalytics
Exceeds Standards
Exceeds US CFR section 21 requirements with rigorous testing for aerobic and anaerobic bacterial species as well as fungi, and yeast.
Comprehensive Reports
Utilizes state-of-the-art MALDI-TOF mass spectrometry to identify hundreds of bacterial and fungal genera, providing comprehensive contamination insights within 11 days.
Inspires Confidence
Reliable microbiology results from a trusted team gives you confidence in your research outcomes and total control over your laboratory safety.
Protects Your Research
Detects microbial contamination that may not be apparent through turbidity or pH changes, protecting against data variability and animal health threats.
Broad Detection
We accept a broad range of samples for sterility testing, including: any materials that have been passaged in animals, cell culture media, lab reagents, airborne contaminant swabs, and other environmental sources.
Sterility Testing sample collection & shipping
![]()
Sample Requirements
Cells, Culture Media, or Other Liquid for Sterility
1000 μL is needed for sterility - please consult with our CSS team if you want to order multiple tests on the same material.
![]()
Collection Instructions
Submit tissue culture media + cellular material as some microorganisms are intracellular or highly cell-associated.
![]()
Shipping
Submit overnight with ice packs to maintain a cool temperature and insulation to prevent freezing.
![]()
Turnaround Time
11 business days
![]()
Additional Information
The following tests can be performed on a single vial of cells: CellCheck, STAT-Myco, h-IMPACT, IMPACT, iCS-Digital, STAT-Endo and ConfirmPDX.
A separate vial of cells is required for sterility testing
Please consult with your account manager if you have any questions
Fast, accurate, and reliable results from the team you know and trust.
Connect with IDEXX BioAnalytics today.